Liquid chromatography–mass spectrometry (LC-MS) is a widely applied analytical technique used to characterize complex mixtures across various disciplines by integrating the separation capabilities of liquid chromatography (LC) with the detection and molecular identification offered by mass spectrometry (MS). LC-MS is frequently applied in pharmaceutical development, food safety, environmental monitoring, and clinical research to identify and quantify chemical compounds at trace levels.
LC-MS systems are available in multiple configurations to meet different analytical requirements. Some are suited for routine, high-throughput workflows, while others are designed for more intricate analyses involving complex matrices or trace-level detection. These systems incorporate various ionization sources—such as electrospray ionization (ESI) or atmospheric pressure chemical ionization (APCI)—to accommodate a broad range of compound types. Paired with optimized chromatography columns, LC-MS enables reliable separation and detection of analytes in both targeted and untargeted workflows.
System selection should reflect the nature of the sample and the level of sensitivity or resolution required. Whether applied in food analysis, pharmaceutical quality control, or biomarker discovery, LC-MS offers versatility and precision across numerous analytical settings.
Types of LC-MS Systems
In liquid chromatography–mass spectrometry (LC-MS), system configurations vary depending on the analytical objective. Each type offers distinct performance characteristics that align with particular workflows, whether for quantitative measurements, structural analysis, or complex mixture characterization.
Ion Trap Mass Spectrometry (MS)
Ion trap mass spectrometers use a combination of radio frequency (RF) and direct current (DC) voltages to generate a three-dimensional electric field that confines ions based on their mass-to-charge ratio (m/z). As the RF voltage increases, ions are destabilized and sequentially released according to their m/z values for detection. These systems can isolate selected ions and fragment them using techniques such as collision-induced dissociation (CID), providing detailed structural information.
Ion traps are well-suited for examining complex mixtures and offer multiple stages of mass analysis in a compact format. Although their mass resolution may be lower than that of time-of-flight (TOF) or Orbitrap systems, they are frequently used in applications that benefit from extensive fragmentation data, such as peptide mapping and metabolite profiling.
Single Quadrupole LC-MS
Single quadrupole LC-MS systems provide a straightforward method for mass-based ion separation and detection. These instruments contain one quadrupole mass analyzer, composed of four parallel metal rods arranged in two opposing pairs. When alternating radio frequency (RF) and direct current (DC) voltages are applied to the rods, an oscillating electric field is generated, allowing ions within a specific mass-to-charge (m/z) range to pass through the analyzer while others are filtered out.
The quadrupole is typically positioned downstream of the liquid chromatography system, where analytes are first separated. After ionization, the filtered ions enter the quadrupole and are directed to the detector based on their m/z values. This design supports consistent performance in targeted workflows and is well-suited for applications where advanced structural data or high-resolution analysis is not required.
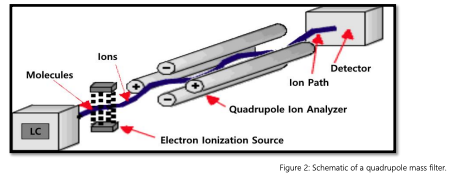
These systems are commonly used for routine compound identification and quantification, particularly in quality control, environmental testing, and pharmaceutical workflows. Their straightforward design supports high-throughput screening and is often selected for laboratories managing defined analytical tasks on limited budgets. While single quadrupole instruments do not support advanced fragmentation or high-resolution mass analysis, they provide reliable data for small molecule detection and are often favored in settings that require rapid turnaround and consistent operation.
Tandem Quadrupole LC-MS/MS
Tandem quadrupole LC-MS/MS systems are designed with two quadrupole mass analyzers separated by a collision cell. This configuration supports workflows that involve targeted analysis and structural investigation at low concentration levels. The first quadrupole filters ions based on their mass-to-charge ratio (m/z), isolating a specific precursor ion. This ion then enters the collision cell, where it undergoes fragmentation, typically through collision-induced dissociation (CID). The resulting fragment ions are passed into the second quadrupole for further separation and detection.
While these systems may involve more advanced setup and operation, the ability to generate detailed fragment ion patterns supports compound identification and confirmation, especially in cases where isobaric or structurally similar compounds are present.
Their performance makes them suitable for laboratories conducting targeted assays that rely on accurate detection of trace-level analytes across complex sample matrices. This approach is often used in applications that require selective quantification and structural confirmation, including pharmacokinetics, drug metabolism studies, and contaminant screening.
Triple Quadrupole
This configuration consists of three quadrupole units arranged in series, each performing a distinct function during analysis. In this design, the first quadrupole (Q1) filters precursor ions based on their mass-to-charge ratio (m/z), selecting a specific ion from the sample for further processing. The selected ion then enters the second quadrupole (Q2), which functions as a collision cell. Here, fragmentation occurs through collision-induced dissociation (CID), producing product ions from the precursor. Q2 does not perform mass analysis but instead provides a controlled space for ion dissociation, allowing for targeted fragmentation. The third quadrupole (Q3) then filters and detects the resulting product ions from Q2, based on their m/z values.
This arrangement supports highly selective workflows, particularly through techniques such as multiple reaction monitoring (MRM), where predefined transitions are monitored for both identification and quantification. Triple quadrupole systems are often selected for tasks such as bioanalytical testing, food safety assessments, and environmental monitoring, where low-abundance compounds must be measured in complex matrices. The multi-stage ion selection and fragmentation process contributes to strong signal-to-noise ratios and consistent quantification across a wide range of analytes.
Quadrupole Time-of-Flight (Q-TOF)
Quadrupole Time-of-Flight (Q-TOF) mass spectrometry combines a quadrupole mass filter with a time-of-flight (TOF) analyzer to support high-resolution and accurate mass measurements. In this configuration, the quadrupole serves as the initial mass selector, isolating precursor ions based on their mass-to-charge ratio (m/z) before they are transferred to the TOF analyzer.
The TOF component measures the time required for ions to travel through a field-free region to the detector. Since ions with different m/z values travel at different velocities, the instrument can calculate their masses with high precision. This setup is frequently applied in workflows that demand detailed molecular characterization, such as proteomics, metabolomics, and compound identification in complex biological or environmental samples.
While the instrumentation may involve higher acquisition and operational costs compared to other mass analyzers, Q-TOF systems are well suited for advanced research applications where high-resolution data and mass accuracy are priorities. Q-TOF systems are known for their high resolving power, fast acquisition speeds, and broad mass range. These features allow for simultaneous analysis of a wide array of compounds, including isobaric or closely related species. Their capability to deliver exact mass measurements supports accurate elemental composition assignments and post-acquisition data mining.
Quadrupole Ion Trap
Quadrupole ion trap mass spectrometers are designed to confine ions within a three-dimensional electric field, allowing them to be held and manipulated for extended analysis. This configuration supports selective ion isolation and fragmentation within the same space, making it suitable for both qualitative assessments and quantitative workflows.
Ions are introduced into the trap and maintained in stable trajectories through a combination of radio frequency and direct current voltages. By adjusting the applied voltages, specific ions can be isolated based on their mass-to-charge ratio (m/z), fragmented, and analyzed sequentially. This setup supports multiple stages of mass analysis without the need for separate physical components, offering flexibility in studying complex mixtures.
Quadrupole ion trap systems are frequently used in applications requiring detailed structural information, such as the analysis of peptides, metabolites, or other biologically derived compounds. Their ability to perform tandem mass spectrometry (MS/MS) within a compact system makes them practical for research in pharmaceutical development, food testing, and biomolecular studies. The design allows for a wide range of analytical tasks without extensive system reconfiguration.
Fourier Transform LC-MS (FT-MS)
Fourier Transform LC-MS (FT-MS) systems are designed to deliver high-resolution and high-accuracy mass measurements by converting ion oscillation frequencies into mass spectra using Fourier Transform algorithms. This approach provides detailed spectral data, allowing for the detection of subtle mass differences between closely related ions.
These systems are often applied in studies where high mass precision is required, such as isotope labeling experiments, proteomics, and structural elucidation of complex molecules. Their ability to resolve ions with minimal mass differences supports applications involving highly complex mixtures or low-abundance analytes.
FT-MS instruments generally involve advanced system architecture and may require a higher level of operator training and maintenance compared to lower-resolution platforms. Due to their sensitivity to experimental conditions and calibration, consistent performance may depend on controlled operational parameters. Despite the added complexity, these systems are frequently used in research environments where high-resolution data is necessary for confident interpretation.
Ionization Techniques
In liquid chromatography–mass spectrometry (LC-MS), ionization methods are responsible for converting neutral analytes into gas-phase ions suitable for mass detection. This process directly affects sensitivity, selectivity, and compatibility with various analyte types. The following section outlines three common ionization approaches—Electrospray Ionization (ESI), Atmospheric Pressure Chemical Ionization (APCI), and Atmospheric Pressure Photoionization (APPI)—each suited to specific compound classes and instrumental configurations.
Electrospray Ionization (ESI)
Electrospray Ionization (ESI) is a popular method in mass spectrometry for ionizing polar and high molecular-weight compounds. In this process, a high voltage is applied to a liquid sample flowing through a narrow capillary, forming a fine aerosol spray of charged droplets. As the solvent gradually evaporates from these droplets, desolvated ions are transferred into the mass analyzer for detection.
This technique is considered a soft ionization method, as it typically preserves the molecular integrity of large, non-volatile compounds such as peptides, proteins, and nucleotides. ESI operates under atmospheric pressure and supports a wide range of solvents, making it suitable for various analytical conditions. It is capable of producing ions in both positive and negative modes, depending on the analyte’s chemical characteristics and the solvent system used.
ESI is often selected for applications involving thermally sensitive or high-mass analytes, due to its ambient-temperature operating conditions. However, the presence of non-volatile salts, buffers, or additives can suppress ionization, reducing sensitivity and complicating data interpretation. Careful sample preparation and buffer selection are recommended when applying this technique in complex matrices.
Atmospheric Pressure Chemical Ionization (APCI)
Atmospheric Pressure Chemical Ionization (APCI) is commonly applied for ionizing compounds with low to moderate polarity where the liquid sample is first vaporized using a heated nebulizer. The resulting gas-phase analytes are then subjected to a corona discharge, which generates ions through gas-phase ion–molecule reactions.
Unlike Electrospray Ionization (ESI), which operates through direct ionization of the liquid phase, APCI relies on vapor-phase interactions, making it more suitable for analytes that require thermal desolvation. It rather supports higher mobile phase flow rates, making it compatible with conventional HPLC systems. When considering APCI for a method, the physical properties of the analyte and its ability to vaporize without degradation should be evaluated.
APCI is particularly effective in workflows involving complex mixtures, where matrix effects and ion suppression must be managed. While it is not typically applied to large biomolecules such as peptides or proteins, it performs consistently with small to medium-sized compounds, including lipids, steroids, and neutral or moderately polar molecules that are not easily ionized in solution. The system's adaptability and stability make it a strong option for applications in pharmaceutical analysis, environmental monitoring, and food testing.
Atmospheric Pressure Photo Ionization (APPI)
Atmospheric Pressure Photoionization (APPI) works by ionizing non-polar or weakly polar compounds that are not easily addressed by Electrospray Ionization (ESI) or Atmospheric Pressure Chemical Ionization (APCI). This method uses a UV lamp to produce photons, which ionize analytes either directly or indirectly through a photoionizable dopant such as toluene or acetone.
Unlike ESI or APCI, APPI does not rely on a charged solvent environment, making it more effective for analytes with low polarity or limited ionization potential in solution. This characteristic allows APPI to address analytes that may not respond well to conventional ionization techniques, particularly in complex matrices where selectivity is required.
APPI accommodates both liquid and solid-phase sample introduction and supports a broad range of solvent systems and flow conditions. This approach provides additional ionization coverage when traditional methods are not suitable, making it a practical choice for laboratories working with chemically diverse analyte profiles. While it is applied less frequently than other ionization methods, it is well suited for specialized workflows in environmental studies, petrochemical testing, and pharmaceutical analysis where neutral or non-polar compounds are present.
Matrix-Assisted Laser Desorption/Ionization (MALDI)
Matrix-assisted laser desorption/ionization (MALDI) is coupled with time-of-flight mass spectrometers for analyzing large biomolecules and polymers. The sample is embedded within an organic matrix compound that absorbs energy from a laser pulse. When irradiated, the matrix vaporizes rapidly, transporting the analyte into the gas phase and ionizing it with limited fragmentation.
MALDI typically produces intact molecular ions, making it suitable for characterizing proteins, peptides, nucleic acids, and high molecular-weight polymers. MALDI provides sensitive detection and rapid sample analysis, though the preparation process can be extensive, involving careful sample and matrix selection and co-crystallization steps.
While MALDI performs well with large molecules, its performance with smaller compounds can be less effective due to matrix-related interference. Nonetheless, MALDI remains widely applied in proteomics, polymer chemistry, and related fields where the analysis of large or complex molecules is routinely conducted.
Hydrophilic Interaction Chromatography Systems (HILIC)
Hydrophilic Interaction Chromatography (HILIC) provides a specialized liquid chromatography approach for the separation of highly polar analytes. The technique combines standard high-performance liquid chromatography (HPLC) practices with a polar stationary phase and a mobile phase of comparatively lower polarity.
HILIC is particularly effective in separating hydrophilic biomolecules such as peptides, nucleotides, carbohydrates, and metabolites, which typically show limited retention under traditional reversed-phase chromatography conditions. The method finds frequent application in proteomics and metabolomics workflows, where detailed mass spectral characterization is required for compound identification.
The compatibility of HILIC with various mass spectrometry systems expands its applicability across diverse analytical tasks, particularly in biological and pharmaceutical research where polar analytes require distinct separation mechanisms.
Disclaimer: The content provided on the Birch Biotech blog is for educational and entertainment purposes only. The information offered here is designed to provide helpful insights and advice related to laboratory practices and supplies.
Readers are advised to refer to our product-specific quality data sheets and Certificates of Analysis (COAs) available on our website for detailed information on product specifications. It is essential to handle and store all materials according to the safety guidelines and regulatory requirements applicable to your area.
While we endeavor to ensure the accuracy and relevance of the information published, it should not be used as a substitute for professional advice or official protocols. We encourage all our readers to consult their institution's guidelines, local regulations, and professional standards before implementing any practices discussed here.
Birch Biotech does not accept liability for any actions undertaken based on the information provided in this blog nor for the misuse of our products. Furthermore, Birch Biotech does not guarantee the completeness, reliability, or timeliness of the information contained on this website.
This disclaimer is subject to change at any time without notifications.
Sources for this Article:
-
https://www.agilent.com/en/product/liquid-chromatography-mass-spectrometry-lc-ms/lcms-fundamentals
-
https://www.acdlabs.com/blog/a-beginners-guide-to-mass-spectrometry-types-of-ionization-techniques/

