The physical and chemical attributes of solvents inherently interact with a sample and analytical instruments, so choosing the one best for your analysis is a key component for obtaining accurate and reliable results. Whether you are working with chromatography or spectroscopy, this "solvent effect" impacts everything from separation efficiency to detection sensitivity. For example, in nuclear magnetic resonance (NMR) spectroscopy, the choice of solvent extraction scheme can significantly impact the spectral clarity of samples. In high-performance liquid chromatography (HPLC), the solvent affects factors such as elution strength and detection performance, influencing the separation efficiency and detection sensitivity of your analysis.
Understanding solvent attributes will help you attain optimized results for unique tests across various industries and purposes. The properties of solvents, such as polarity, viscosity, and UV absorbance, directly influence the quality and precision of your analytical data. By carefully considering these properties, you can enhance the resolution and accuracy of your results across various analytical techniques and applications.
Roles of Solvents in Chemical Reactions
In a chemical analysis, solvents influence reaction rates, solubility, and product yields. They facilitate chemical reactions by dissolving reactants and products, providing a medium for molecules to interact (affecting reaction rates and equilibria). Some actively participate in reactions, while others can act as reactants, catalysts, or even stabilize intermediate species.
In chemical syntheses, solvents can act as heat transfer agents, controlling temperature and preventing localized overheating. In organometallic chemistry, coordinating solvents can affect catalyst activity directly. Solvents also influence selectivity in organic reactions, for they can promote specific reaction pathways and lead to different product distributions. For example, protic solvents often favor SN1 reactions, while aprotic solvents often promote SN2 reactions.
Understanding Solvent Polarity
Solvent polarity is one of the most important factors in determining solubility and reactivity. Polar solvents like water and alcohols have relatively high dielectric constants, suited for dissolving ionic compounds. Non-polar solvents such as hexane or toluene are better suited for dissolving non-polar organic molecules.
Polarity affects the solvation of reactants and products. Highly polar solvents can stabilize charged species, influencing reaction rates and equilibria. In contrast, non-polar solvents may promote aggregation of polar molecules.
In chromatography, solvent polarity affects retention times and peak separation. For mass spectrometry, solvent selection can influence ionization efficiency and spectral quality.
The polarity index is a relative scale used to rank solvents based on their polarity (P'). A higher P' value relates to a higher polarity for the compound. The polarity index is not directly measured but is a semi-empirical assignment from properties such as dielectric constant, dipole moment, and hydrogen bonding ability. For example, water has a polarity index of around 10, methanol 5.1, and hexane 0.1, reflecting their relative polarities:
|
Solvent |
Polarity Index (P') |
|---|---|
|
Water |
10.2 |
|
Dimethyl Sulfoxide |
7.2 |
|
Acetonitrile |
5.8 |
|
Methanol |
5.1 |
|
Acetone |
5.1 |
|
Ethyl Acetate |
4.4 |
|
Chloroform |
4.1 |
|
Tetrahydrofuran |
4.0 |
|
Isopropyl Alcohol |
3.9 |
|
Dichloromethane |
3.1 |
|
Chlorobenzene |
2.7 |
|
Toluene |
2.4 |
|
Hexane |
0.1 |
Classification of Solvents
Solvents can be categorized based on their molecular properties and behavior. These classifications help predict solvent interactions and guide solvent selection for various analytical processes.
Polar Versus Non-Polar Solvents
Polar solvents have an uneven distribution of electrical charge within their molecules, creating a permanent dipole. Water is a classic example of a polar solvent. Non-polar compounds, like carbon tetrachloride, lack this charge separation:
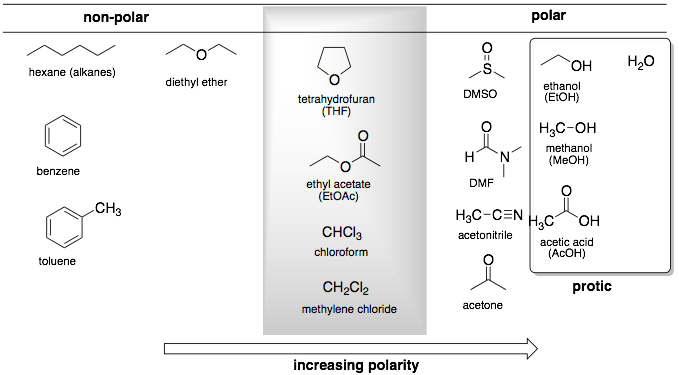
Polarity affects a solvent's ability to dissolve different substances. Polar solvents excel at dissolving ionic compounds and other polar molecules. Non-polar solvents are better suited for dissolving non-polar substances like oils and fats.
When choosing a solvent for your analysis, consider the polarity of both the solvent and the analyte. The general rule "like dissolves like" applies here: polar solvents work best with polar analytes, and non-polar solvents with non-polar analytes.
Protic Versus Aprotic Solvents
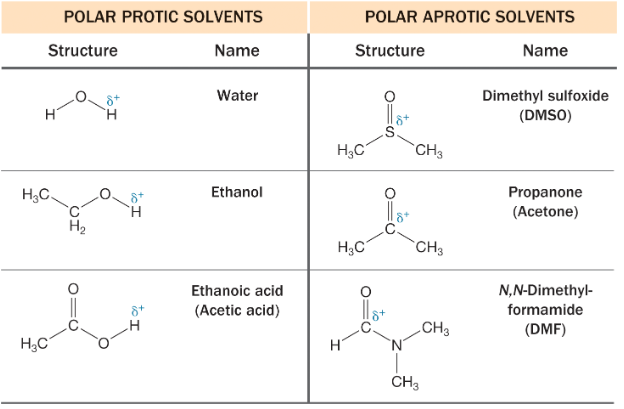
Understanding the difference between protic and aprotic solvents is central to controlling reaction behavior and optimizing analytical conditions. Solvent choice can influence reaction kinetics, equilibria, solubility, and detection—particularly in environments where hydrogen bonding or polarity plays a role.
Protic solvents contain hydrogen atoms bonded to electronegative atoms such as oxygen or nitrogen. These hydrogen atoms can participate in hydrogen bonding with solutes, which may affect reactivity and solvation behavior. The presence of these interactions can either facilitate or interfere with certain chemical pathways, depending on the analyte and reaction mechanism.
Common protic solvents include water, methanol, ethanol, and other alcohols. Their ability to donate hydrogen bonds influences solubility for ionic or polar compounds and can affect outcomes in both synthetic and analytical applications.
Aprotic solvents, by contrast, do not possess hydrogen atoms that can serve as hydrogen bond donors. However, many of these solvents contain electronegative atoms with lone pairs—such as oxygen or nitrogen—that allow them to act as hydrogen bond acceptors. Examples include acetone, acetonitrile, dimethyl sulfoxide (DMSO), and ethyl acetate.
The absence of donor hydrogen atoms makes polar aprotic solvents well-suited for reactions or analyses where hydrogen bonding needs to be minimized. Their dipole moment and solvation properties often support faster reaction rates for nucleophilic substitutions and greater analyte stability in certain detection systems.
Organic Versus Inorganic Solvents
Organic solvents contain carbon atoms and are derived from living matter or synthesized from organic compounds. Common organic solvents include ethanol, acetone, and toluene.
Inorganic solvents do not contain carbon-hydrogen bonds. Water is the most common inorganic solvent. Other examples include liquid ammonia and sulfuric acid.
This classification is important when considering solvent properties like boiling point, flammability, and toxicity. Organic solvents generally have lower boiling points and are more flammable than inorganic ones.
The choice between organic and inorganic solvents can affect sample preparation, extraction efficiency, and instrument compatibility in analytical procedures.
Solvents and UV Absorbance
Solvents affect UV spectroscopy in two primary ways. First, their polarity and other properties can alter the absorption characteristics of analytes, leading to shifts in peak wavelength or intensity (solvatochromic effects). Second, solvents themselves can absorb UV light, potentially interfering with analyte detection. To avoid this, it is crucial to choose a solvent with minimal absorbance in the wavelength range of interest and calibrate the detection wavelength for the analyte in the given solvent to ensure accurate measurements without obscured peaks.
The UV cutoff wavelength λ of absorption for several common solvents is charted below. This wavelength indicates the minimum wavelength at which the solvent begins to strongly absorb UV light. Below this wavelength, the solvent’s absorbance becomes significant, potentially interfering with UV detection. Solvents are typically chosen to have a UV cutoff lower than the wavelength range of interest to avoid interference. Wavelengths between 200 - 400 nm impact UV radiation while 400 - 800 nm is within the visible light spectrum:
|
Solvent |
λ of absorption |
|---|---|
|
Water |
191 nm |
|
Ether |
215 nm |
|
Methanol |
203 nm |
|
Ethanol |
204 nm |
|
Chloroform |
237 nm |
|
Carbon tetrachloride |
265 nm |
|
Benzene |
280 nm |
|
Tetrahydrofuran |
220 nm |
Suitable solvents are transparent at the wavelength region you're investigating. The solvent's cutoff wavelength determines the lower limit of your spectral range.
Different Properties Affecting UV
Polar solvents can shift absorption peaks based on the functional groups and chemical properties of the analyte, known as solvatochromic effects. . Polar solvents may cause hypsochromic shifts (blue shifts) in compounds with non-bonding lone pairs, such as on amines or carbonyls. The polar solvent stabilizes the ground state n→π* transitions in these functional groups of the analyte, resulting in a lower UV absorption wavelength (higher energy) needed for excitation.
Polar solvents can cause bathochromic shifts (red shifts) in compounds with conjugated double bonds and aromatic rings due to stabilized π→π* transitions in the conjugated double bonds of the analyte by the polar solvent. This occurs due to the stabilization of the ground and excited states of the molecule.
The dielectric constant of the solvent further influences the extent of solvatochromic effects of UV absorption. The higher the dielectric of the solvent, the more stabilized polar and changed groups are in the solution. This has the effect of increasing the intensity of bathochromic shifts in analytes with π→π* transitions while decreasing any hypsochromic shifts in analytes with n→π* transitions.
The buffer solution used can also affect your results. Ensure your buffer doesn't absorb light in your measurement range, as this can lead to artificially high absorbance values.
Physical Properties of Solvents
A solvent's physical characteristics influence how it interacts with samples and testing environments, shaping the behavior of analytes and the outcome of analytical procedures. Understanding these properties helps guide solvent selection based on the requirements of each method.
Boiling Point and Its Significance
The boiling point of a solvent represents the temperature at which it transitions from liquid to vapor under atmospheric pressure. This parameter influences volatility, heat tolerance, and suitability for thermal processes.
Solvents with higher boiling points tend to evaporate slowly and are preferred in workflows involving prolonged heating, such as reflux systems or methods requiring consistent solvent presence over time.
Conversely, solvents with lower boiling points evaporate more rapidly, which can be beneficial in procedures like extractions or concentration steps. These may, however, require containment strategies to minimize solvent loss or sample variability during processing.
Solvent selection tools can help you compare boiling points and other properties when choosing the right solvent for your needs.
Flash Point and Safety Concerns
The flash point is the lowest temperature at which a solvent produces enough vapor to form a flammable mixture with air that can ignite when exposed to an ignition source. This property is paramount for laboratory safety and proper handling.
Solvents with low flash points are highly flammable and require extra precautions:
-
Use only in well-ventilated areas
-
Keep away from heat sources and open flames
-
Store in appropriate safety cabinets
Higher flash point solvents are generally safer to handle but may still pose risks. Always consult the safety data sheet (SDS) for specific handling instructions.
When working with volatile organic compounds, be aware of their flash points to prevent accidental fires or explosions in your laboratory.
Evaporation Rate and Its Impact
The evaporation rate of a solvent affects sample preparation and can affect analysis when using techniques like MS. This property is often compared to the evaporation rate of butyl acetate, which is assigned a value of 1.
Fast-evaporating solvents (high evaporation rate):
-
Quickly concentrate samples
-
Useful for rapid solvent removal
-
May lead to sample loss if not carefully controlled
Slow-evaporating solvents (low evaporation rate):
-
Provide more stable conditions during the analysis
-
Allow for longer processing times
-
May require additional time for solvent removal
When selecting a solvent, consider how its evaporation rate might affect your sample preparation and analytical procedure. For instance, in gas chromatography, evaporation differences between sample, solvent, or carrier gas can impact peak shape and retention times. Likewise, in HPLC, evaporation of the mobile phase from the reservoir could affect mobile phase polarity and separation efficiency.
You can look into detailed physical characteristics of solvents, including evaporation rates, to help you make informed decisions for your analyses.
Viscosity of Solvents with Analytes
Solvent viscosity is particularly important in liquid chromatography. It affects flow rates, mixing efficiency, and overall system performance. Understanding these impacts can help you optimize your analytical methods.
How Viscosity Affects Liquid Chromatography
In liquid chromatography, solvent viscosity indirectly influences separation efficiency and resolution by affecting system parameters such as flow rate, column pressure, and mass transfer efficiency. Lower viscosity solvents like methanol and acetonitrile enhance system resolution and stability. They allow for better flow through columns and improved mixing with analytes.
Higher viscosity solvents can lead to increased back pressure in your system. This may require adjustments to your flow rates or column selection. It can also impact the diffusion of analytes, affecting peak shapes and separation quality.
The relationship between solvent viscosity and analyte diffusion is described by the Stokes-Einstein equation:
D = kT / (6πηr)
Where:
-
D is the diffusion coefficient
-
k is Boltzmann's constant
-
T is temperature
-
η is solvent viscosity
-
r is the radius of the analyte molecule
This equation indicates that as solvent viscosity (η) increases, the diffusion coefficient (D) decreases, meaning analytes move more slowly through the solvent. In chromatography, reduced diffusion limits the mass transfer of analytes between the mobile and stationary phases. This can lead to band broadening and reduced separation efficiency in your chromatographic analysis.
When selecting solvents for LC or MS methods, consider their viscosity carefully. Balancing viscosity with other solvent properties can help you achieve optimal separation and detection of your analytes.
Solvents in Industrial and Laboratory Settings
Solvents are widely used in multiple industrial sectors for their ability to dissolve, extract, clean, or carry substances. In dry cleaning, solvents remove soil and stains from fabrics without the need for water-based washing. In the coatings and paint industries, solvent mixtures dissolve pigments and resins to support application and drying.
Automotive processes often rely on solvent blends for degreasing engine components and cleaning metal surfaces. In electronics manufacturing, solvents are applied in the cleaning of printed circuit boards and precision components. Pharmaceutical manufacturing uses solvents throughout drug synthesis, purification, and formulation stages.
Glycol ethers are commonly found in cleaning agents, coatings, and ink formulations. Their amphipathic structure allows them to interact with both polar and non-polar compounds, offering broad solubility across diverse material types. Though they share some characteristics with surfactants, they do not form micellar structures in solution.
Analytical Techniques Affected by Solvents
Solvents influence the performance and reproducibility of many analytical systems. In High-Performance Liquid Chromatography (HPLC), solvents serve as the mobile phase and affect both separation and elution behavior. Careful selection based on polarity, viscosity, and UV transparency supports consistent analyte detection.
Liquid Chromatography–Mass Spectrometry (LC-MS) depends on solvents that facilitate ionization and minimize suppression effects. Solvent compatibility with both the stationary phase and mass spectrometer directly influences detection efficiency and resolution.
In spectroscopy, solvent characteristics must be matched to analytical needs. Solution-state Nuclear Magnetic Resonance (NMR) often requires deuterated solvents to minimize background signals. Infrared (IR) spectroscopy demands solvents with minimal absorbance in regions overlapping with analyte functional groups.
Gas Chromatography (GC) relies on solvent volatility and polarity for sample preparation and introduction. The properties of the injection solvent affect peak symmetry, retention time, and baseline quality.
Safe Practices for Solvent Handling
Solvent use requires attention to safe handling, particularly due to risks related to flammability, inhalation, and environmental release. Storage in properly rated containers and well-ventilated spaces reduces the likelihood of exposure or accidents.
Personal protective equipment (PPE) is recommended when working with solvents. This may include gloves, lab coats, safety glasses, closed footwear, and, where necessary, respiratory protection based on volatility and exposure potential.
Spent solvents should be collected in clearly labeled containers, segregated by class or compatibility, and managed according to applicable waste disposal guidelines. Failure to manage waste properly may lead to contamination or regulatory issues.
Monitoring workplace exposure levels can help maintain a safe environment. Regulatory bodies have established permissible exposure limits (PELs) for many commonly used solvents, which can guide ventilation design and protective practices in both laboratory and production settings.
Managing Solvent Waste and Residue
Handling solvent waste with care reduces environmental contamination. Solvents should not be discarded via drainage systems or released into the air. A structured waste protocol should be established to manage disposal and recovery.
Recommended practices include:
-
Separating incompatible solvent types
-
Using clearly labeled containers for storage
-
Coordinating routine collection through certified waste management services
Recovery methods such as distillation and filtration may allow reuse of certain solvents, particularly when applied in large volumes or high-frequency processes. These techniques can reduce waste generation and lower the cost of solvent use over time.
When recovery is not feasible, incineration under controlled conditions may be applied. Proper emissions management during this process helps limit secondary environmental impacts.
Where feasible, the use of lower-toxicity or bio-based solvents may reduce health risks and minimize environmental persistence.
Disclaimer: The content provided on the Birch Biotech blog is for educational and entertainment purposes only. The information offered here is designed to provide helpful insights and advice related to laboratory practices and supplies.
Readers are advised to refer to our product-specific quality data sheets and Certificates of Analysis (COAs) available on our website for detailed information on product specifications. It is essential to handle and store all materials according to the safety guidelines and regulatory requirements applicable to your area.
While we endeavor to ensure the accuracy and relevance of the information published, it should not be used as a substitute for professional advice or official protocols. We encourage all our readers to consult their institution's guidelines, local regulations, and professional standards before implementing any practices discussed here.
Birch Biotech does not accept liability for any actions undertaken based on the information provided in this blog nor for the misuse of our products. Furthermore, Birch Biotech does not guarantee the completeness, reliability, or timeliness of the information contained on this website.
This disclaimer is subject to change at any time without notifications.
Sources for this Article:
-
https://chemistry.mdma.ch/hiveboard/picproxie_docs/000532906-14581_17.pdf
-
https://diverdi.colostate.edu/all_courses/CRC%20reference%20data/UV%20solvents.pdf
-
https://jchps.com/specialissues/Special%20issue3/06%20jchps%20si3%20nanni%2049-52.pdf
-
https://nerd.wwnorton.com/ebooks/epub/karty2/EPUB/content/15-chapter02-09.xhtml
-
https://www.sciencedirect.com/science/article/pii/S0023643824014385
-
https://shimizu-uofsc.net/orgo/kb/knowledge-base/classification-of-solvents/
-
https://www.smacgigworld.com/blog/factors-affecting-uv-vis-spectroscopy.php
-
https://www.wiredchemist.com/chemistry/data/physical_character_solvents.html

