Chromatography offers a combination of technologies to separate, analyze, and protect samples from contaminants. Various operational considerations, like selecting suitable columns and understanding the principles behind each technique, enhance experimental outcomes. This is why understanding the different types of columns and their functions is key when exploring the field of chromatography.
Chromatographic columns house the stationary phase and are essential for separating and analyzing individual compounds in a sample. They are typically packed with stationary phases made of silica, polymer-based materials, or chemically bonded phases (e.g., C18 for reversed-phase chromatography). The column’s design, including particle size, pore structure, and stationary phase chemistry, influences separation efficiency, resolution, and purification capabilities. Size-exclusion chromatography (SEC) is a liquid chromatography technique commonly used to separate large molecules, such as proteins and polymers, based on their size. In contrast, gas chromatography (GC) relies on factors like carrier gas selection, column chemistry and diameter, and temperature control to achieve efficient separation of volatile compounds. Different types of columns are used in both HPLC and GC, each tailored for specific applications ranging from pharmaceutical analysis to industrial quality control. Your column choice, whether for liquid chromatography or gas chromatography, significantly impacts the separation process. By exploring this article, you will gain insights into how chromatography columns work and how they can improve analytical and purification methods in practical applications.
Fundamentals of Columns and Stationary Phases
Chromatography is a technique used to separate components of a mixture based on their differential interactions with a stationary and a mobile phase. A typical chromatography system consists of several key elements. The stationary phase can be a solid like silica or a liquid immobilized on a solid support. In liquid chromatography, the liquid mobile phase solvates and carries the sample through the column packed with the stationary phase. In gas chromatography, the inlet volatilizes a sample, and a carrier gas mobile phase moves the volatilized sample through the column containing the stationary phase. Each component plays a role in the separation and analysis process.
Principles of Separation
Chromatography separates mixtures by exploiting the different affinities of individual components for the stationary and mobile phases. As the mixture passes through the column, sample components are retained by the stationary phase based on their differential interactions, with compounds interacting strongly with the stationary phase taking longer to elute. This differential interaction results in different paths and elution speeds for each compound, effectively separating them. Larger molecules may move more slowly, and this is the main separation principle employed by techniques like size exclusion chromatography, where particles are separated by size.
Stationary Phase Characteristics
The stationary phase is the immobile component where separation occurs. It can vary in material, such as functionalized silica or polymer materials, and is selected based on the requirements of the experiment. Its surface area, pore size, and surface chemistry are critical for effective separation. This phase must interact appropriately with sample components to achieve the desired separation. Columns can be packed with particles or used in a capillary format, which impacts the behavior of the chromatography system.
It's essential to determine the differences between a column and the stationary phase:
-
A chromatography column is the physical structure, typically a tube or container, that holds the stationary phase. These columns can be constructed from glass, metal, or plastic and are specifically designed to aid in the separation process used in techniques such as liquid chromatography (LC) or gas chromatography.
-
The stationary phase refers to the material within the column that engages with the sample components and mobile phase, facilitating their separation according to various properties, including polarity, size, charge, or affinity. This phase can consist of a solid, such as silica or polymer beads, or a solid coated with a liquid.
In the diagram below, you can see just how a column is packed with a stationary phase for a sample preparation:
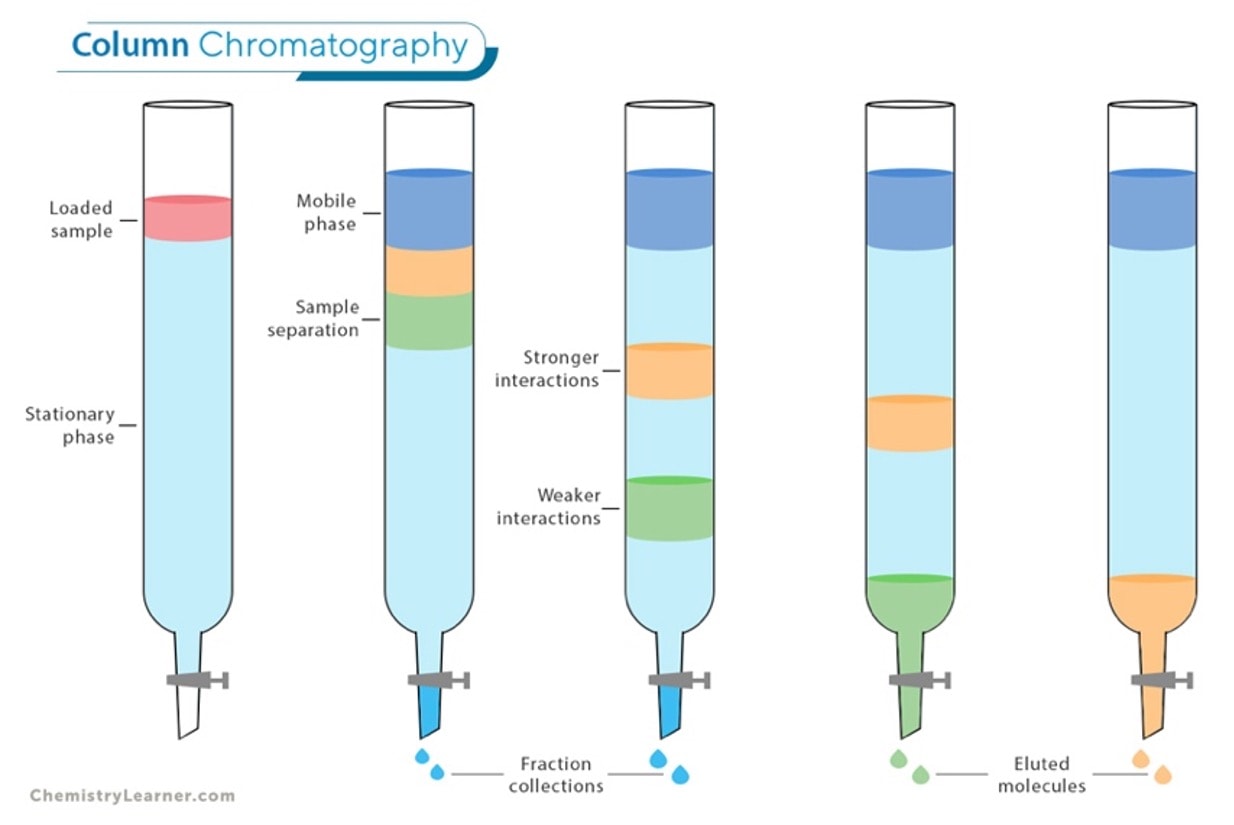
A stationary phase is usually composed of a porous solid such as silica gel, alumina, or polymeric resin. This material is packed into a column and serves as a stable surface on which different components of the mixture can interact with and separate based on their polarity or other chemical properties during chromatography. This controlled interaction with the stationary phase allows for the differential retention of analytes, enabling their separation.
Mobile Phase Roles
The mobile phase carries the sample through the stationary phase. Its composition and flow rate significantly influence the separation process. In HPLC, solvents must be pure and tailored to the specific analytical requirements. In GC columns, the selection of carrier gas impacts the efficiency and selectivity of the separation. The mobile phase needs to be compatible with the stationary phase to maintain system stability and ensure accurate analysis.
Chromatography Columns
Chromatography columns are fundamental components in various separation techniques, vital for analyzing and examining intricate mixtures. They are available in a wide array of sizes, materials, and applications, each meticulously crafted to fulfill distinct requirements and optimize the separation process. It's important to consider the stationary phase material, such as silica, for your particular application.
Column Selection Criteria
When selecting a chromatography column, consider both the type of separation needed and the sample’s properties. Key factors include the analyte's chemistry, the desired separation efficiency, and compatibility with the mobile phase. Choose a column that maximizes resolution while maintaining optimal analyte retention. Additionally, particle and pore sizes are crucial for effective separation, especially for larger biomolecules and polymers.
Size, Scale, and Material Variations
Chromatography columns come in various sizes to accommodate different application scales, ranging from analytical to preparative and industrial volumes. In analytical lab-scale liquid chromatography, these columns generally measure 25 to 150 cm in length with an average of 4 mm in internal diameter and are often made of stainless steel. Gas chromatography (GC) columns vary based on design and application. Packed GC columns typically range from 1 to 3 meters in length and 2 to 4 mm in internal diameter, with stainless steel or glass construction. In contrast, capillary GC columns are much longer, typically ranging from 15 to 60 meters, with narrow internal diameters of 0.1 to 0.53 mm, and are made from fused silica with a polyimide coating.
Analytical GC columns are designed for high-resolution separation and are typically long and narrow, maximizing efficiency for detailed analysis. While larger-diameter packed columns allow for higher sample loads, gas chromatography is primarily used as an analytical technique rather than for preparative-scale separations.
The material of a chromatography column influences its durability. Stainless steel and glass are popular choices, each with its own specific advantages. Stainless steel is known for its durability and is commonly used in high-pressure applications, while glass is more inert, enables visual inspection, and is appropriate for low-pressure processes.
A column must consist of materials strong and chemically resistant enough to endure both operational pressures and the chemical effects of the mobile phase. While most columns are constructed from stainless-steel tubing, some low-pressure applications may also utilize heavy-walled glass. Commercially available columns can withstand a wide range of pressures, from low-pressure glass columns to ultra-high-pressure stainless steel columns exceeding 15,000 psi in UHPLC applications.
In high-pressure applications, PEEK-lined stainless-steel columns may be used for enhanced chemical resistance, particularly with bio-inert or highly reactive analytes. Minimizing dead volume in column end fittings is essential to prevent peak broadening and ensure optimal separation efficiency. Porous frits at both ends retain the stationary phase while allowing the mobile phase to flow through efficiently. Liquid chromatography columns are frequently favored in straight sections. Some columns may be configured in a “U” shape, while coiled columns can be used; however, they typically offer lower efficiency than straight sections.
Pre-columns are also advantageous. They help condition the mobile phase and sample before entering the analytical column, reducing separation inconsistencies. They also protect the main column by trapping contaminants and removing particulates that could interfere with the analysis.
Stationary Phases
Stationary phases serve a pivotal role in chromatography, significantly impacting the separation and efficiency of analytes. These phases may be silica-based or polymer-based, each presenting distinct properties and applications. The surface chemistry and functionalization of the stationary phase can further augment their selectivity and performance.
Silica-Based Phases
Silica-based phases are widely utilized in liquid chromatography. Their porous structure provides a high surface area, enhancing analyte retention and interaction. Silica phases offer high mechanical stability and broad solvent compatibility, making them suitable for a wide range of chromatographic techniques.
The surface of silica can be modified to enhance selectivity. For instance, functionalizing the surface with C18 groups creates a non-polar surface, which is ideal for separating non-polar compounds in reversed-phase HPLC. These modifications allow for customizing the phase to meet specific separation requirements. Silica-based phases generally provide high separation efficiency and are frequently used in analytical applications.
Polymer-Based Phases
Polymer-based phases are widely used in liquid chromatography and in some cases, gas chromatography. They are frequently selected for their stability across a wide pH range and their resistance to aqueous environments. These phases are composed of materials such as polystyrene or polymethacrylate, which can be functionalized to enhance selectivity.
Due to their flexible pore structures, polymer-based phases can be particularly useful for larger molecules, such as proteins and polysaccharides. The diversity in functional group attachments allows you to explore a wide range of separation techniques. Such adaptability makes polymer-based phases a valuable component in chromatography, especially when traditional silica phases are unsuitable.
Surface Chemistry and Functionalization
Surface chemistry is pivotal for controlling the selectivity and efficiency of separation through stationary phases. Modifying the chemical groups on the surface can enhance the interaction with the target sample components, increasing retention time and separation efficiency. Depending on the intended application, this functionalization can involve hydrophilic or hydrophobic modifications.
Functionalization enhances separation and broadens the range of applications. For example, incorporating ionic functional groups enables ion-exchange chromatography by selectively retaining charged analytes. Modifying the surface chemistry serves as a strategic tactic to optimize performance and address complex separation needs across diverse fields. With meticulous selection and development, functionalized phases improve chromatographic techniques.
Analytical Techniques
Grasping analytical techniques in chromatography requires exploring how samples are introduced, detected, and analyzed. These processes dictate the efficiency and effectiveness of separating and identifying substances.
Sample Introduction and Handling
Properly introducing a sample into a liquid chromatography column is the first step for achieving optimal column performance. The sample should be introduced as a well-focused band at the column inlet to ensure optimal resolution. If the sample is not properly focused during injection, peak broadening occurs, reducing separation efficiency at the column’s output. In gas chromatography, the sample is injected through a septum using a micro-syringe into a heated inlet system. In liquid chromatography, the sample is typically introduced using an autosampler or loop injector to ensure consistent injection volumes.
Packing Material
HPLC columns typically contain either superficially porous (core-shell) or fully porous particles. Core-shell particles, which have nearly replaced older pellicular particles, consist of solid cores coated with a thin, porous outer layer of silica or polymer-based materials. These particles, typically ranging from 2 to 5 µm in diameter, enhance separation efficiency by reducing band broadening while maintaining lower back pressure compared to fully porous particles of similar size. In contrast, fully porous particles, which remain widely used, range from 1.7 to 10 µm in diameter and are primarily composed of silica or polymer-based resins. Among these, silica is the most prevalent type of packing material in modern HPLC applications.
Bonded-phase HPLC employs columns in which the stationary phase is chemically bonded to hydrolyzed silica particles. The separation mechanism depends on the functional groups attached to the silica surface, which dictate interactions with analytes. Common bonded phases include alkyl chains for reversed-phase chromatography, polar functional groups for normal-phase, and charged moieties for ion-exchange chromatography. The table below lists R groups functionalized onto silica and their typical chromatographic applications.
|
R Group Attached to Siloxane |
Chromatography Method |
|
Alkyl |
Reverse Phase |
|
Fluoroalkyl |
Reverse Phase |
|
Cyano |
Normal and Reverse Phase |
|
Amide |
Reverse Phase |
|
Amino |
Normal and Reverse Phase |
|
Dimethylamine |
Weak Anion Exchanger |
|
Quaternary Amine |
Strong Anion Exchanger |
|
Sulfonic Acid |
Strong Anion Exchanger |
|
Carboxylic Acid |
Weak Cation Exchanger |
|
Diol |
Reverse Phase |
|
Phenyl |
Reverse Phase |
|
Carbamate |
Reverse Phase |
Packing a Chromatography Column
While packing a column, it is important to first determine the choice between a dry and wet packing method:
-
Dry packing: This approach first fills a column with a dry solid phase, followed by a controlled introduction of a mobile phase to ensure even packing. It is more common in low-pressure chromatography.
-
Wet packing: Requires suspending the stationary phase in the mobile phase to form a slurry, which is then packed into the column, often under high pressure, to create a uniform bed.
Wet packing is typically the preferred method in most scenarios due to its efficiency in utilizing less liquid and its overall time-saving advantages over dry packing. Regardless of the chosen approach for filling the column, it is essential to ensure that the stationary bed is uniformly level and devoid of bubbles.
From here, the following column packing steps are typically completed for best results:
-
Level the column prior to packing.
-
Wet it, then drain the bottom column frit with a buffer for 30-60 seconds.
-
Plug the column outlet, leaving 1-2 cm buffer at the bottom column.
-
Re-suspend the resin slurry to assure homogeneity, often by use of an ultrasonic bath.
-
Pour the resin slurry along the column wall to prevent air-trapping.
-
After transferring the resin slurry to the column, rinse its inside walls with a squirt bottle of packing buffer.
-
Open the outlet and start the pump slowly to buffer the flow through the column.
-
Gradually increase the flow rate to avoid hydraulic shock and uneven packing in the column bed. Adjustments should depend on the column size and target rate, with examples provided in the table below:
|
Column Size |
Target Flow Rate |
Increment |
Hold Time |
|
2.2 cm x 60 cm |
2 mL/min |
0.5 mL/min |
0.5 min |
|
9 cm x 30 cm |
300 mL/min |
50 mL/min |
2 min |
|
25 cm x 30 cm |
2,000 mL/min |
400 mL/min |
3 min |
-
After the bed has fully formed, shut off the pump and close the column outlet.
-
Position the bed in the lower column for a packing reservoir.
-
Siphon the supernatant from the upper reservoir using a pipette or pump.
-
Remove the upper reservoir and coupling ring.
-
Place the flow adapter 2-3 cm from the consolidated bed. Avoid introducing air column.
-
Secure the adapter, start the pump again, and open the column outlet.
-
Stop the pump and close the column once compression is complete and pressure stabilizes.
-
Gently release the flow adapter seal and bring it near the resin bed without disturbing it.
-
Repeat until no more resin bed compression occurs from the flow adapter. Typically, this takes 2-3 iterations to stabilize.
-
Finally, lower the adapter 1 – 5 mm into the bed.
Running a Sample Through a Column
Once the column is packed and equilibrated with the mobile phase, the sample is introduced via an injection port (for HPLC) or carefully loaded onto the column using a syringe (for low-pressure chromatography). Following the sample introduction, flow is initiated either by applying pump pressure (HPLC) or by opening the column outlet (low-pressure chromatography).
Depending on the separation mechanism, analytes either adsorb to the stationary phase, partition into the mobile phase, or diffuse through a porous matrix to initiate separation. Maintaining proper mobile phase conditions is essential, as disruptions to partitioning equilibrium or improper column equilibration can negatively impact resolution.
The separation process is contingent upon the properties of the molecules involved and the degree of their interactions with the stationary phase. In more straightforward terms, an analyte that exhibits strong interactions with the stationary phase is retained within the column, resulting in a slower movement through it. Conversely, when the interactions are weak, the analyte can elute with greater ease, consequently exiting the column first. These variations in elution speed separate the components of a mixture (see below).
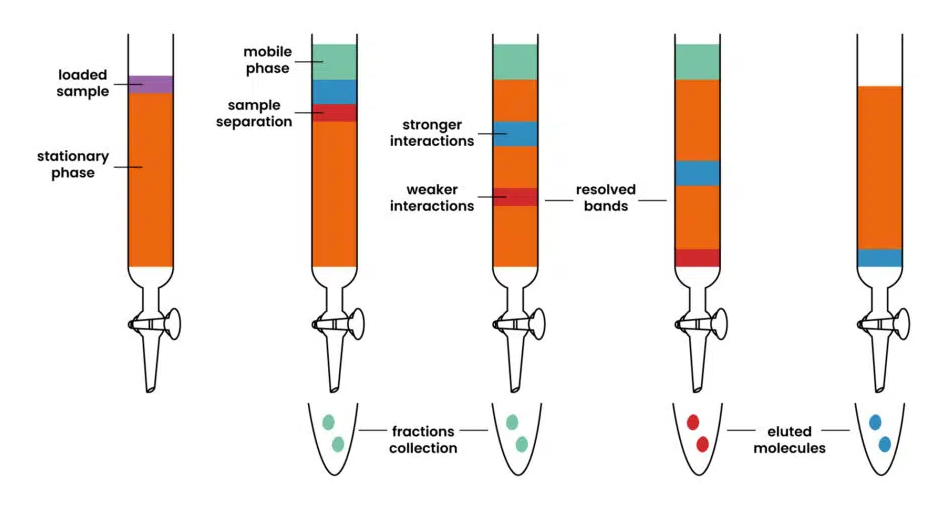
Detection Methods & Data Collection
After running a sample through a correctly prepped column with the processes outlined above, you should be ready for analyte detection and data collection.
Detection in chromatography involves measuring the presence and concentration of separated components within a mixture. Common detection methods include UV-Vis absorbance detection, fluorescence detection, and mass spectrometry, each selected based on the properties of the analytes. In gas chromatography, flame ionization detectors are typically used for organic compounds. Each technique offers distinct advantages, particularly regarding sensitivity and selectivity. For example, mass spectrometry can provide comprehensive molecular data that is especially beneficial for analyzing complex mixtures. The choice of detection method should align with the chemical and physical characteristics of the components to ensure accurate measurement.
Data analysis is a key part of chromatography, allowing you to interpret the results derived from detection methods. Software applications assist in the processing of chromatograms to ascertain retention times and peak areas. These parameters are utilized to identify and quantify the components of the sample. Calibration curves may be requisite for precise quantification. Furthermore, awareness of potential contaminants is of paramount importance. A comprehensive understanding of the principles of data analysis can augment the reliability of the results, thereby facilitating improved decision-making in experimental contexts.
Types of Chromatography with Columns
Chromatography includes various techniques for isolating components of a mixture. The selection of a method varies based on the sample's characteristics, the intended separation, and its application. In this section, we delve into three key types: Liquid Chromatography, Gas Chromatography, and Size Exclusion Chromatography.
Liquid Chromatography (LC)
High-Performance Liquid Chromatography (HPLC) columns are engineered for high precision and speed, making them highly sought after in the pharmaceutical and life sciences industries. HPLC columns generally utilize silica-based packing materials and come in various pore sizes to accommodate different analyte dimensions, although other stationary phase materials can be used depending on the application. Choose stationary phases that promote efficient mass transfer and high-strength materials to offer robust mechanical strength to endure the operational pressures common in HPLC systems.
Liquid Chromatography (LC) is a flexible technique frequently utilized in areas such as pharmaceuticals and environmental testing. In this method, a liquid mobile phase transports the sample through a column filled with a stationary phase. The interactions between the stationary phase, the sample's components, and the mobile phase influence their separation and retention times. A popular variant of LC is High-Performance Liquid Chromatography (HPLC), known for its high resolution and rapid performance.
HPLC uses high pressure to drive solvents through columns filled with small particle stationary phases. This technique is preferred for the analysis and purification of intricate mixtures. Different stationary phases, including functionalized silicas and polymers, can improve separation by utilizing various properties depending on the desired application and sample.
Gas Chromatography (GC) Columns
Gas Chromatography (GC) columns employ gases such as helium or nitrogen as the mobile phase to carry the sample through the column. Capillary columns are notable types of GC columns, recognized for their narrow diameter and high efficiency. In most cases, a thin film of liquid stationary phase is coated or chemically bonded to the inner surface, which improves selectivity. When selecting GC columns, consider your specific separation needs, including temperature stability and resolution requirements, and ensure that the carrier gas fits your application.
Gas chromatographic columns can be over 60 meters in length for capillary columns. The liquid stationary phase is either bonded or adsorbed to the inner surface of an open tubular (capillary) column, or it is placed on a packed solid support within the column. Selecting an appropriate stationary phase based on analyte polarity is a key factor in achieving effective separation. For optimal retention, the analyte should have an appropriate level of interaction with the stationary phase, depending on the chromatographic mode. The thickness of the stationary phase in capillary columns varies from 0.1 to 8 µm. Thicker stationary phase films (e.g., 3–8 µm) enhance the retention of highly volatile analytes by increasing their interaction time with the stationary phase, while thinner films (e.g., 0.1–0.25 µm) are preferred for less volatile compounds to reduce peak broadening.
Components are categorized by their volatility and interaction with the stationary phase. This method is utilized in environmental testing and contaminant detection. Capillary columns with a narrow diameter enhance efficiency, while those with a larger diameter accommodate higher sample loads.
Size Exclusion Chromatography (SEC) Columns
Size Exclusion Chromatography (SEC) effectively separates molecules based on their size using porous beads. The stationary phase is usually made from materials such as agarose or dextran. Smaller molecules are delayed as they enter the pores, while larger molecules move through more quickly. This technique is frequently used in polymer analysis and protein purification.
SEC enables the analysis of complex mixtures without causing destructive interactions. The selection of bead size and pore diameter can greatly impact the separation process. This method is beneficial for examining biological macromolecules in both research and development.
Zeolites are commonly used as molecular sieves. These sieves contain pores that allow small molecules to enter while larger molecules cannot. This design enables larger molecules to pass through the column more quickly than smaller ones because they are not held up by adsorption in the zeolite. Other packing materials for size exclusion chromatography include polysaccharides, various polymers, and silica. The pore sizes for size exclusion separations range from 4 to 200 nm.
Ion Exchange Chromatographic Columns
Ion exchange columns facilitate the separation of ions and easily ionizable molecules. The effectiveness of this separation depends on the ion's affinity for the stationary phase within the ion exchange system. Electrostatic interactions among the analytes, mobile phase, and stationary phase play a crucial role in separating ions from the sample. Only complexes with either positive or negative charges can engage with their respective cation or anion exchangers. Common ion exchange resins are composed of cross-linked polystyrene-divinylbenzene, often functionalized with sulfonic acid groups (for cation exchange) or quaternary amines (for anion exchange).. Early ion exchangers were often inorganic, primarily composed of aluminosilicates (zeolites). However, aluminosilicates are now infrequently utilized as ion exchange resins.
Chiral Columns
Chiral columns are employed to separate enantiomers. The separation of chiral molecules relies on stereochemistry. These columns feature a stationary phase that selectively interacts with one enantiomer over the other. Such columns are invaluable for separating racemic mixtures. The table below outlines various stationary phases used for enantiomer separation, along with their associated chromatographic properties methods.
|
Stationary Phase |
Method(s) Used |
|
Metal Chelates |
GC, LC |
|
Amino Acid Derivatives |
GC, LC |
|
Proteins |
LC |
|
Helical Polymers |
LC |
|
Cyclodextrin Derivatives |
GC, LC |
Applications of Chromatography Columns
Chromatography is used in various fields because it can separate and analyze the components of a mixture. From purifying substances for pharmaceuticals to monitoring environmental pollutants, chromatography allows for a detailed examination and analysis of materials.
Purification of Substances
Chromatography is crucial in purifying and analyzing substances, particularly in the pharmaceutical industry. It facilitates the separation of impurities from active ingredients, which is especially useful when handling complex mixtures that require the isolation of specific components. Techniques such as liquid chromatography are commonly employed to ensure precise separation and high-quality products. HPLC columns are widely used because they efficiently separate components based on their differential interactions between the mobile phase and the stationary phase.
Substances like polymers or polysaccharides are frequently purified using chromatography to achieve the desired level of purity. This process is also effective in removing contaminants from different types of samples. The selection of the stationary phase and mobile phase can substantially affect the results, tailoring to the specific requirements of the purification process.
Life Sciences and Biotechnology
Chromatography is used in life sciences to analyze biological molecules such as proteins and DNA. Size exclusion chromatography enables the separation of larger molecules from smaller ones. This method helps analyze polymers, assess protein folding, and study complex carbohydrates.
Chromatography also promotes the advancement of new biotechnologies. It enables the separation and analysis of biomolecules, helping researchers study molecular interactions such as protein-ligand binding, enzymatic activity, and metabolic pathways. By providing detailed molecular profiles, chromatography contributes to understanding biological functions, disease mechanisms, and drug interactions. By providing detailed analyses, chromatography enhances the research and development phase in biotechnology.
Environmental Monitoring
Environmental monitoring benefits from gas chromatography's ability to detect pollutants in air and water. GC columns analyze complex mixtures, identifying harmful substances such as pesticides and volatile organic compounds. Choosing an appropriate carrier gas and column stationary phase is essential for achieving precise analysis.
Chromatographic techniques offer valuable insights into pollution levels, assisting regulatory bodies in developing action plans to reduce environmental risks. Monitoring air quality and water contaminants becomes more efficient, enabling informed decisions to safeguard the environment.
Quality Control in Industry
Chromatography is a fundamental tool for quality control and assurance in various industries. It verifies product conformity to specifications and detects impurities. This is especially important in the food and beverage industry, where HPLC ensures products are safe for consumption by verifying the absence of harmful substances.
The petrochemical and pharmaceutical sectors also benefit from these techniques. Chromatography ensures product purity and consistency, adhering to industry standards. Through detailed analysis, chromatography helps optimize production processes and improve product quality by identifying and controlling potential variances.
Operational Considerations
Successfully managing chromatography operations involves maintaining the system, improving efficiency, and following important safety procedures. By focusing on these areas, you can enhance the reliability and accuracy of your results during chromatography experiments.
System Maintenance
Regular maintenance of chromatography equipment can significantly enhance performance and longevity. Ensure that columns are correctly stored, and lines are regularly flushed to remove the buildup of contaminants. It is beneficial to periodically check and replace GC columns along with other consumables like filters, septum, liners, and frits.
Monitoring the mobile phase reservoirs and cleaning injection systems prevent potential blockages in liquid chromatography. Record-keeping for service activities helps track maintenance schedules and anticipate future needs, keeping your chromatography system in top operating condition.
Troubleshooting and Optimization
To optimize chromatography operations, monitor peak shape and retention time. If irregularities such as peak tailing or broadening occur, these may indicate issues with column packing, system pressure, or mobile phase composition in liquid chromatography. Adjusting the flow rate and mobile phase composition can resolve separation challenges.
Evaluate if the stationary phase is suitable for your specific sample components, ensuring the column accommodates the size and nature of the molecules being separated. Optimizing parameters, therefore, leads to better separation and faster analysis times, increasing the efficiency of your processes.
Safety and Best Practices
Safety protocols are fundamental in chromatography to protect both the user and the equipment. All chemicals used in the mobile phase should be handled with care, using appropriate PPE and proper ventilation. Additionally, the system should be regularly inspected for leaks, particularly when working with gases like hydrogen in gas chromatography, which burns with a near-invisible flame.
Follow manufacturer guidelines for equipment setup and operation and ensure that all system handlers are well-trained. By adopting these practices, you will ensure the safety and reliability of your chromatography experiments.
Sources for this Article:
-
https://www.chemistrylearner.com/chromatography/column-chromatography
-
https://egyankosh.ac.in/bitstream/123456789/43309/1/Unit-5.pdf
-
https://www.sciencedirect.com/science/article/abs/pii/B9780323999687000072
Disclaimer: The content provided on the Birch Biotech blog is for educational and entertainment purposes only. The information offered here is designed to provide helpful insights and advice related to laboratory practices and supplies.
Readers are advised to refer to our product-specific quality data sheets and Certificates of Analysis (COAs) available on our website for detailed information on product specifications. It is essential to handle and store all materials according to the safety guidelines and regulatory requirements applicable to your area.
While we endeavor to ensure the accuracy and relevance of the information published, it should not be used as a substitute for professional advice or official protocols. We encourage all our readers to consult their institution's guidelines, local regulations, and professional standards before implementing any practices discussed here.
Birch Biotech does not accept liability for any actions undertaken based on the information provided in this blog nor for the misuse of our products. Furthermore, Birch Biotech does not guarantee the completeness, reliability, or timeliness of the information contained on this website.
This disclaimer is subject to change at any time without notifications.








